HANSCOM AIR FORCE BASE, Mass. – The Department of Defense is leveraging Air Force Life Cycle Management Center acquisition professionals for their rapid contracting expertise to boost national stockpiles of critical medical equipment and supplies in the fight against COVID-19.
Acting in concert with the Department of Health and Human Services and the Federal Emergency Management Agency, officials from the Digital Directorate, headquartered here, sourced over $123 million from the Defense Production Act, and other relief legislation, on six contracts to increase domestic manufacturing of nasal cannulas, swabsticks, and other COVID-19 testing supplies.
“Early on, we established a number of teams standing ready to rapidly respond to this challenge and expand U.S. production capacity for medical supplies,” said Steven Wert, program executive officer Digital. “We conducted streamlined acquisition strategy panels on these efforts in order to apply the lessons learned from one to the next as we tackled these efforts.”
On March 25, the DOD established the COVID-19 Joint Acquisition Task Force, also known as JATF. In response, the Dept. of the Air Force stood up its Acquisition COVID-19 Task Force, or DAF-ACT.
To execute directives from these task forces, Digital established a number of small teams focused on rapid acquisitions and contracting. During the pandemic, these teams have provided liaison support to DAF-ACT leadership, conducted market research to align 24 critical medical requirements to possible vendors, and awarded contracts, primarily in the defense industrial base expansion line of effort.
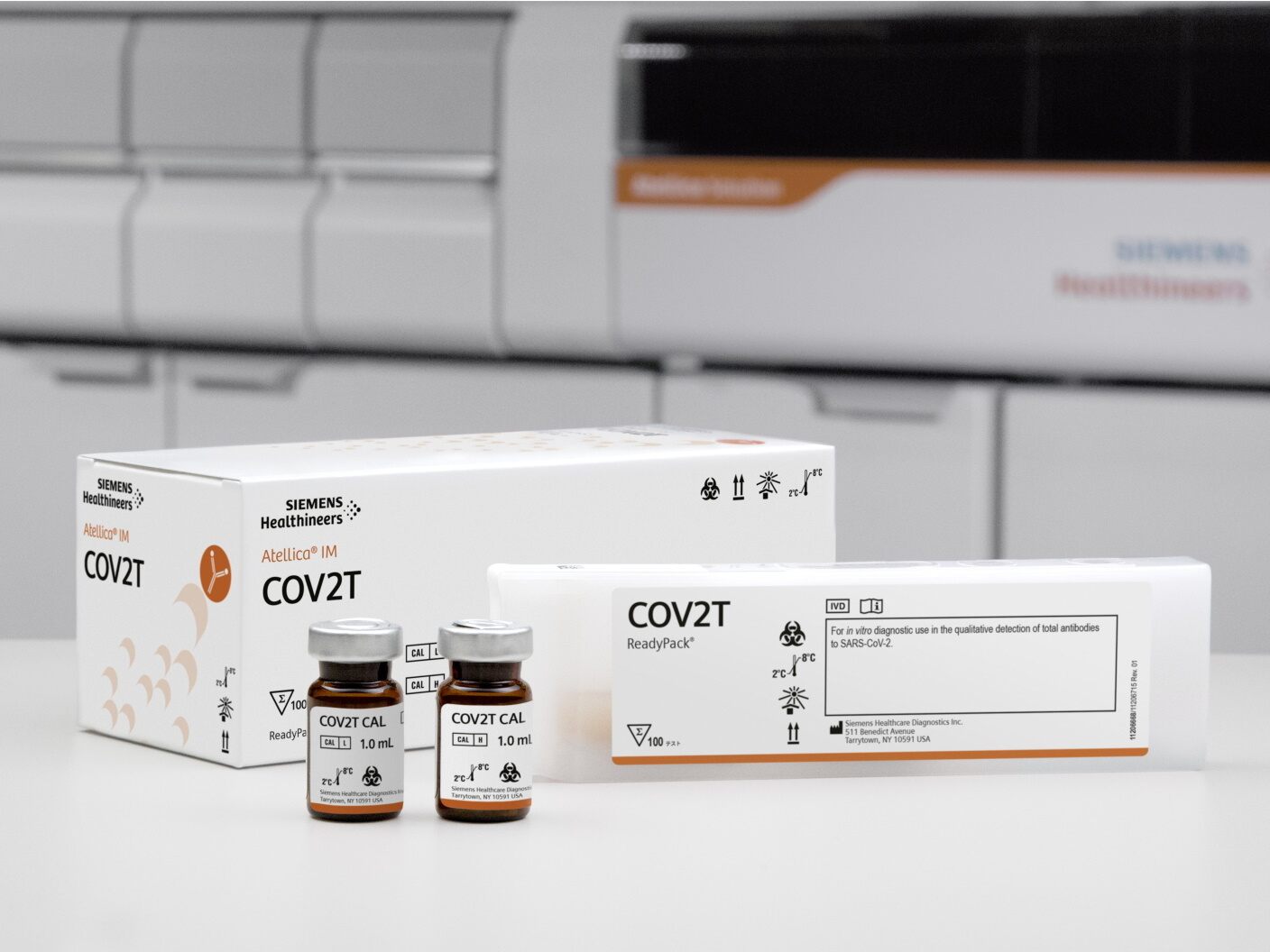
$12.38 million contract with Siemens Healthineers for SARS-CoV-2 antigen test kits
One of these teams, made up of members of the Command, Control, Intelligence, Surveillance and Reconnaissance Division, awarded a $12.38 million agreement Nov. 19 to Siemens Healthineers for increased domestic production of their SARS-CoV-2 antigen test kits at their Walpole, Massachusetts, manufacturing facility. Siemens current production capacity is 8.25 million kits. However, Capt. Ryan Torno, program manager, Distributed Common Ground System, Global Network Systems, expects that number to increase to 50 million kits per month by July 2021.
“This test is critical in both rapidly identifying an active infection in a patient and assisting in vaccine development,” said Torno. “Our team is working to ensure that we deliver this capability to the nation as quickly as possible to aid in both current and future pandemic response and recovery efforts.”
$10 million contract with Vapotherm for nasal cannula therapy systems
Another small team within the Airborne Warning and Control System Division utilized their rapid acquisition expertise to award a decentralized $10 million blanket purchase agreement with medical device company Vapotherm in late May.
Vapotherm, a publicly held corporation based in Exeter, New Hampshire, is the only U.S. Food and Drug Administration-approved manufacturer of high-velocity, high-flow nasal cannula therapy systems, a treatment for acute respiratory distress brought on by COVID-19.
Under the terms of the agreement, 51 military treatment facilities across the U.S. have the ability to purchase directly from the company at a rate of 17,000 units per month. Prior to the agreement, Vapotherm was only capable of producing 2,400 of the therapy systems per month.
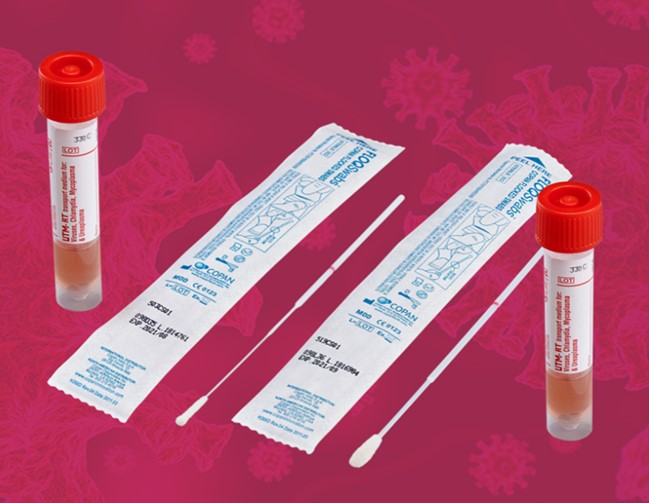
$10 million contract with Copan Industries, Inc. for flocked tip nasal swaps
In September, the team followed up with a $10 million commercial competitive contract with Copan Industries Inc. to boost the national stockpile of flocked tip nasal swabs. Under the terms of the contract, Copan moved production lines from their headquarters in Brescia, Italy to Puerto Rico, as well as modified their facility to accommodate expanded production capacity.
Lt. Brock Koleski, materiel leader of the Advanced Capabilities branch, states that the manufacturer is currently producing nearly 3.6 million flocked swabs per week.
$33 million contract with Tecan Group Ltd. for disposable pipette tips
Members of the Theater Battle Control Division also joined in on defense industrial base expansion efforts by entering into a $33 million contract with medical supply company Tecan Group Ltd. in late October for the U.S. production of disposable pipette tips for fully automated diagnostic COVID-19 testing.
The contract with Tecan, which sources funding from the Healthcare Enhancement Act of 2020, will allow the Swiss company to establish four production lines in Wisconsin, as well as hire additional employees, said Gina Hubbard, deputy chief, Theater Battle Control Division.
Signed into law April 24, the Healthcare Enhancement Act of 2020 provided $484 billion in funding to replenish and supplement key programs under the Coronavirus Aid, Relief, and Economic Security Act.

$6.98 million contract with Teel Plastics, LLC for COVID-19 testing swabsticks
In an effort to expand U.S. production capacity of two types of COVID-19 testing swabsticks, members of the Strategic Warning and Surveillance Systems Division awarded a $6.98 million firm-fixed-price contract to Teel Plastics, LLC on Oct. 16.
Peter Knudsen, chief of the Operations Management branch, said the agreement will increase production from 70 million units per month to over 165 million per month by March 2021.
“With our highly motivated team, we were able to rocket through this effort and get a contract within a month of being tasked and eight days from receiving funding, said Knudsen. “Knowing that this is a part of the overall national effort was a strong motivator.”
$51 million contract with Puritan Medical for flocked tips nasal swabs
Another small team is also working to boost the national stockpile of flocked tip nasal swabs. In early July, the Foreign Military Sales Division began contracting and managing an industrial base expansion program to increase the domestic production of flocked nasal swabs with Puritan Medical of Guilford, Maine. Utilizing CARES Act funding, the team awarded a $51 million contract to Puritan in late July.
“Puritan is making good progress toward meeting their required March 31, 2021, delivery date, which will provide the nation with critical ammunition against the spread of COVID-19, as well as sufficient domestic capacity and supply to face future pandemics,” said Tim Roche, acquisitions branch chief, FMS and International Programs.
With production underway, Roche expects Puritan to increase its monthly output from five to 50 million flock tip swabs.
“These teams deserve a great deal of credit for going above and beyond their already mission-essential duties,” said Wert. “It’s great to see teams at Hanscom, Robins, Peterson, and Tinker Air Force Bases all having the chance to exercise their acquisition muscles in unique and rewarding ways.”
Officials from the Command, Control, Communications, Intelligence and Networks Directorate, headquartered here, are also working to boost U.S. manufacturing of COVID-19 related medical supplies. Visit https://www.hanscom.af.mil/News/ in the coming weeks for updates on their effort.